To listen to Jag and Paul from the Pharmacy Support Helpdesk discuss the content of this section, please scroll down to the bottom of the page.
UPDATE: Wholesale Dealing Guidance |
Please find an updated version of the Wholesale Dealing Guidance with this edition of The Professional Standard. Please discard of any previous versions that you have in store.
IMPORTANT: Patient Safety First |

Included in this month’s edition of The Professional Standard are copies of the updated Monthly Patient Safety Review and associated guidance on its completion. A number of Patient Safety Champions and their teams trialled the use of this form last month and, in the Patient Safety Champions’ letter, their insights and top tips on optimising the use of this important patient safety tool are highlighted.
Also in this month’s letter is a section on the upcoming Winter Flu Vaccination Service. This highlights several key documents and processes the pharmacy team must be aware of to support your vaccinators in keeping patients safe.
PIERS survey |
We are in the process of reviewing our incident reporting forms on PIERS and we welcome your feedback, so that we can look to incorporate this into any changes that we make. If you would like to give us feedback, please complete the Microsoft forms survey (which can be accessed via the following link: https://forms.office.com/r/Am9AHmWb36) by 30 September 2022. Please note that completion of this form is optional and all answers will be anonymous.
IMPORTANT: Buprenorphine - bioavailability of different formats for oral use |
Buprenorphine for oral use – licensed as substitution therapy for opioid drug dependence within a framework of medical, social and psychological treatment – is available in two different formats: sublingual tablets and freeze-dried wafers (Espranor™ Oral Lyosphilate 2mg and 8mg). These two different formats are not interchangeable.
Espranor™ Oral Lyosphilate has a higher bioavailability than the sublingual tablet format of buprenorphine and must be placed ON the tongue until dispersed, which usually occurs within 15 seconds, and is then absorbed through the oral mucosa. Swallowing should be avoided for two minutes.
Please ensure that all pharmacy team members are familiar with the different formats of buprenorphine for oral use, and that each patient is provided with appropriate counselling on how to use their prescribed format correctly, including when doses are supervised.
REMINDER: Pharmacy services document retention |
It is essential that any paper records generated as part of a pharmacy service, including completed consent forms for NHS services and customer record forms for PGD services, are retained in store for the required length of time stipulated on the Pharmacy Document Retention Guidance document. This can be accessed by visiting https://nsp.bootslive.co.uk/sites/bootslive/AllUsers/Pages/Core-Filing-System-and-record-retention-periods.aspx.
All paper records for pharmacy services must be stored in a secure location but must be accessible should they be required by a patient who wishes (as part of a Data Subject Access Request) to obtain a copy of their personal data held by the pharmacy.
REMINDER: Scotland - permitted items for services |
Pharmacy team members in Scotland are reminded of the importance of ensuring that any item(s) supplied via the Patient Group Direction (PGD) for Urgent Provision of Current Prescribed Medicines, Appliances and ACBS Products is allowed under the terms of this PGD. All items that are listed in part B of the PGD schedules (e.g. all strengths of morphine, including Oramorph™ 10mg/5ml Oral Solution), or which are not allowed on an NHS prescription, are excluded from provision via the PGD. Should a UCF electronic claim for an excluded item be received by NHS NSS for processing, the claim will be rejected, and no payment will be made. If a patient requires an urgent supply of a medicine that is not permitted via this PGD, you can still consider making an emergency supply, if appropriate.
Pharmacy team members are also reminded that products supplied under NHS Pharmacy First Scotland should be from the approved list for this service. GSL and P medicines must be used within their licensed indication(s) and the POM products listed must only be used under the terms of the relevant PGDs.
Winter flu jab service |
This year, a number of stores will operate as a Flu Vaccination Centre (FVC) or will follow the Pop-up Plus (PUP) model in which the service is delivered from a location that is often away from the dispensary area. Pharmacists who are working as the Responsible Pharmacist (RP) in these stores should familiarise themselves with the relevant ‘Guidance for Responsible Pharmacists’ (FVC and/or PUP) document on BootsLive (available via: https://nsp.bootslive.co.uk/sites/bootslive/AllUKNonOpticiansUsers/Pages/ Winter-Flu-Hub---Flu-Vaccination-Centres.aspx), which gives an overview of how these sites operate. The guidance also details how a governance framework, in the form of an RP Governance Checklist, serves to ensure that the RP on each day can delegate responsibility confidently to the pharmacist working in the FVC or PUP location.
New vaccinator roles to support delivery of the Winter Flu Jab Service
This year, we will be introducing several different vaccinator roles. In some stores in England, locum nurses from selected agencies will be supporting the delivery of NHS flu vaccinations only. Trainee pharmacists (who are due to qualify in August 2023) and nominated registered pharmacy technicians will be able to administer flu vaccines to customers who access the private service via the DPS route ONLY, in accordance with a pre-dispensed prescription from the Independent Medical Agency. If a customer has switched to either the private PGD or NHS service, the consultation must then be completed by a pharmacist.* This is because trainee pharmacists and pharmacy technicians are not currently listed as authorised healthcare professionals who can supply and/or administer a POM under a PGD. If an appropriate pharmacist is not available,* the appointment must not proceed and should be rescheduled.
Guidance on how to support a locum nurse who is scheduled to work in your store can be found on the ‘People Guidance’ page within the BootsLive Winter Flu Hub: https://nsp.bootslive.co.uk/ sites/bootslive/AllUKNonOpticiansUsers/Pages/Winter-Flu-Hub.aspx.
The ‘Guidance for Winter Flu Service Pharmacist and Vaccinator Planning’ document details the circumstances in which each vaccinator type can support in the different vaccination service delivery models and can also be accessed from this BootsLive page.
*Locum nurses may also administer vaccines under the NHS Flu Vaccination Service.
Ensuring the correct site for an IM injection |
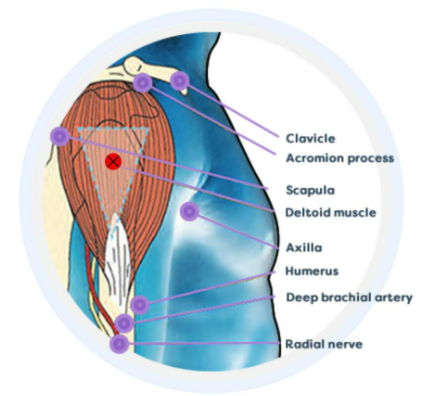
Shoulder injury related to vaccine administration (SIRVA) is a rare event that may occur if an injection is administered into the shoulder joint. This is characterised by an inflammatory process that may cause damage to the musculoskeletal structures, including the bursae, tendons and ligaments. An IM injection that is administered too low in the upper arm or too far to the front or back carries the risk of the vaccine not being placed correctly into the vascularised muscle tissue. There is also a risk of hitting a nerve present in the area.
A video is available to support the training you have done on vaccine administration. The video covers several important aspects of vaccine administration, including how to ensure the deltoid muscle is located correctly, and is available at https://youtu.be/_iwCCZI4oqY.
IMPORTANT: Records for Medicines Support Tool Reviews |
As the review of your patients needing additional support with their medicines moves to a ‘business as usual’ approach (ensure you are familiar with the BootsLive communication issued on 11 August 2022 about this), pharmacy team members are reminded of the requirement to ensure that a full and clear record of the conversations that have been held with patients and/or their carers is documented on the Medicines Support Tool. For each patient concerned, this record should cover a minimum of two conversations; the initial review of the patient’s medicines support needs and the second, follow up conversation to establish how the patient is coping with any new medicines support solution(s) they have been recommended to use.
Completed Medicines Support Tools must be retained in store for three years.
